Attention IVD manufacturers! There’s important news regarding EU MDR/IVDR compliance deadlines. On July 9th, 2024, Regulation (EU) 2024/1860 was published in the Official Journal of the European Union (OJEU). This new regulation offers some key benefits for IVD manufacturers:
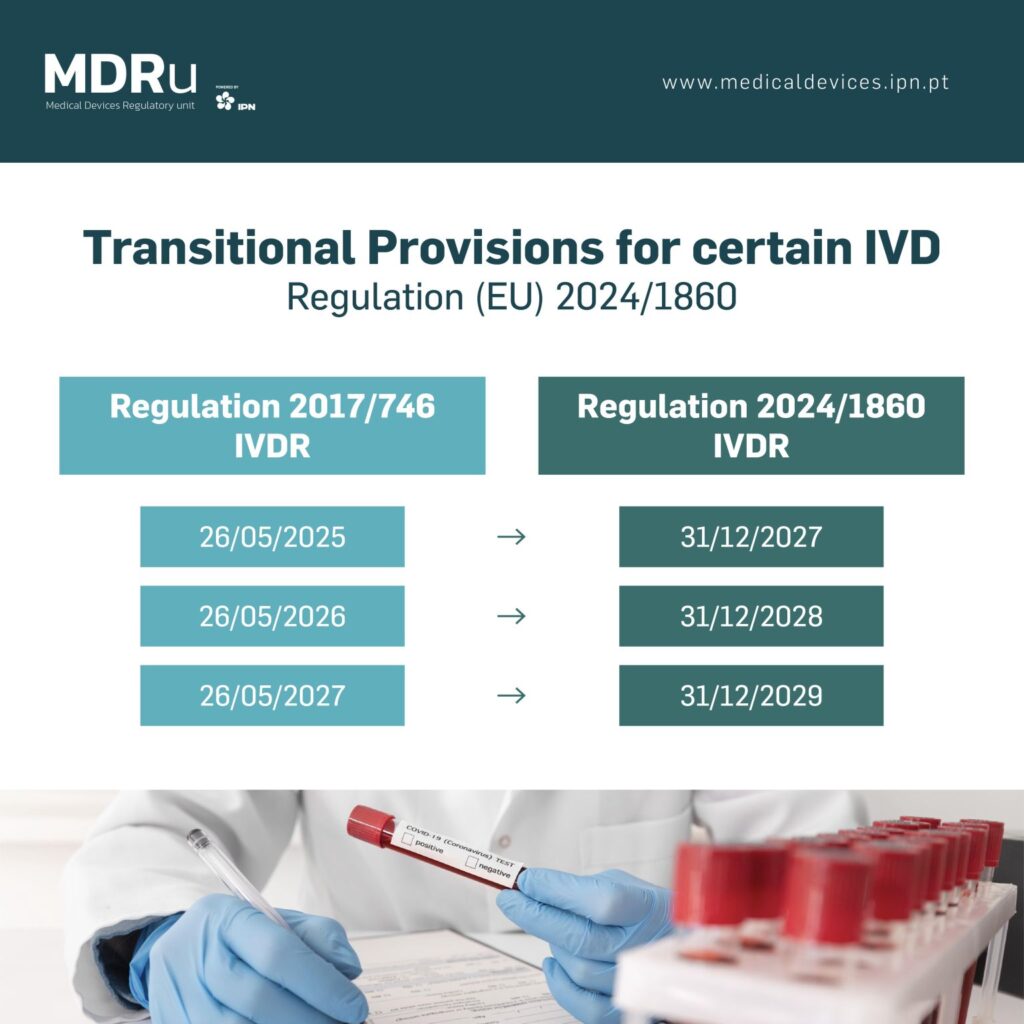
Extended Transition Periods:
– Class D devices now have until December 31st, 2027, to comply.
– Class C devices have an extended deadline of December 31st, 2028.
– Class B devices and sterile Class A devices have until December 31st, 2029.
Phased Rollout of EUDAMED: The new EUDAMED electronic database will be implemented gradually.
Improved Supply Chain Transparency: Manufacturers will be required to report potential shortages of critical devices and IVDs.
This is great news for IVD manufacturers facing challenges meeting the initial deadlines. These extensions provide valuable time to ensure compliance.
Here are some key dates to remember:
– Regulation enters into force: Upon publication in the OJEU (July 9th, 2024)
– Obligations for interruption/discontinuation reporting: January 10th, 2025
Don’t miss out on these extended deadlines! Review Regulation (EU) 2024/1860 and ensure a smooth transition for your IVD products.
Medical Devices Regulatory — support unit (MDRu) can help you comply with Regulation (EU) 2024/1860. Contact us now: https://lnkd.in/dreiWTp7