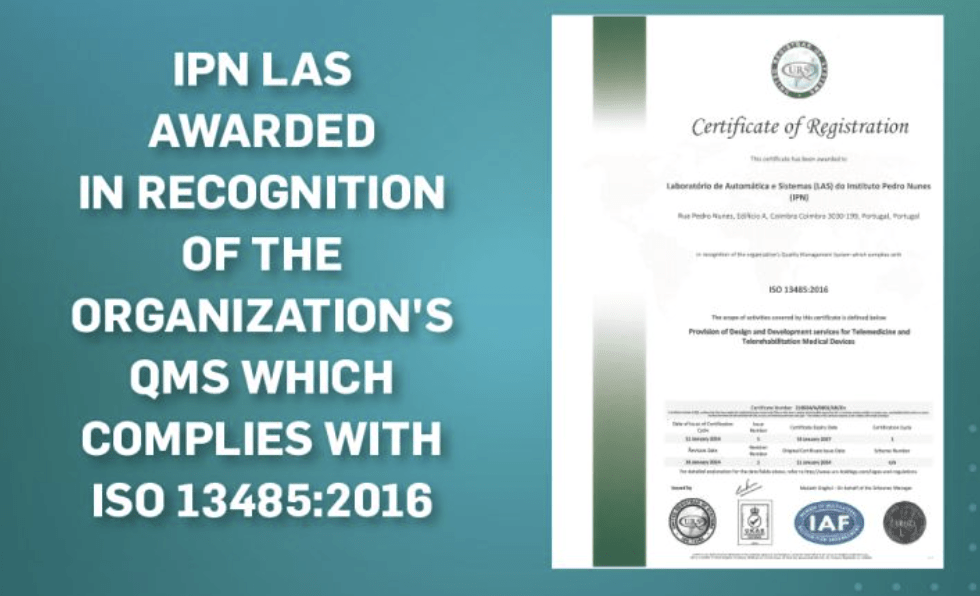
IPN LAS has achieved ISO 13485 certification
Instituto Pedro Nunes Automatics and Systems Lab has achieved ISO 13485 certification, reinforcing our commitment to excellence and quality in the medical devices industry. ISO 13485 certification demonstrates our adherence to the highest standards of quality management systems specifically designed for manufacturers and suppliers of medical devices. This certification also aids our ability to consistently provide services that fulfill regulatory requirements and exceed customer expectations We look forward to continuing our mission of making a positive impact on the healthcare sector.
IPN LAS has achieved ISO 13485 certification Read More »